One of the most common illnesses affecting children under the age of 2 is respiratory syncytial virus (RSV). According to the CDC, as many as 80,000 children in the U.S. are hospitalized for RSV-related health concerns each year.
A new drug approved by the FDA has the potential to reduce the number of infants and young children sickened by RSV. Beyfortus, which was developed by AstraZeneca and Sanofi, is administered in a single shot and helps to reduce the risk of RSV infection.
LeKeyah Wilson, MD, is a pediatrician with Rochester Regional Health and explains the concerns with RSV – and the promise that the newly-approved shot brings for young children and their families.
RSV: The basics
The most common form of RSV is RSV bronchiolitis – a viral respiratory illness characterized by serious respiratory distress. Infants and children often show symptoms of RSV with a runny nose, cough, and fever – and can develop labored breathing.
RSV is a seasonal illness, tending to start in the fall and peak during the winter months. The virus is transmitted between people have close contact with one another.
Currently, there is no designated treatment for RSV; providers can only administer fluids and oxygen if hospitalization is required. Most children can be managed with supportive care measures at home.
Eligibility, side effects & risk factors
The FDA approved Beyfortus for infants and toddlers up to 24 months. The drug, generically called nirsevimab, is administered as a single shot and helps the body’s immune system learn how to fight off harmful pathogens such as viruses.
Other countries, including Canada and several nations in Europe, already use this drug as a way to prevent RSV in young children.
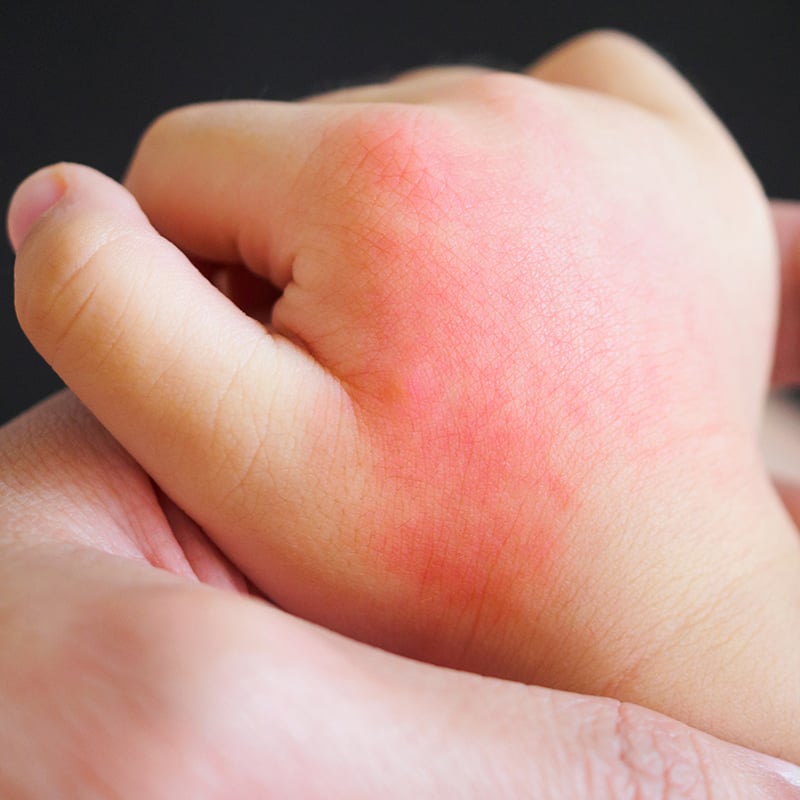
During clinical trials, researchers said the most common side effects were a mild rash or reaction at the spot on the skin where the injection was given. Parents should consult with their pediatrician before having their child receive the shot if they have a history of serious hypersensitivity reactions to any of the active or inactive ingredients in the shot.
“Having the FDA approve this new drug is a significant change in the way we approach preventative care with our littlest patients whose immune systems are often not developed enough to fight an RSV infection well,” Dr. Wilson said.
Earlier this year, the FDA approved an RSV vaccine for adults ages 60 and older. A preventative RSV vaccine created by Pfizer that is administered to pregnant people in a single dose between their 32nd and 36th week of pregnancy also received FDA approval.